Yong Bae Kim M.D. Principal Investigator, "SABR-ROC" Clinical Trial
Yonsei Cancer Center, Seoul, South Korea 
As the principal investigator of the clinical trial "SABR-ROC" for recurrent ovarian cancer, I am writing to extend an invitation to potential participants who may be interested in contributing to this important research endeavor.
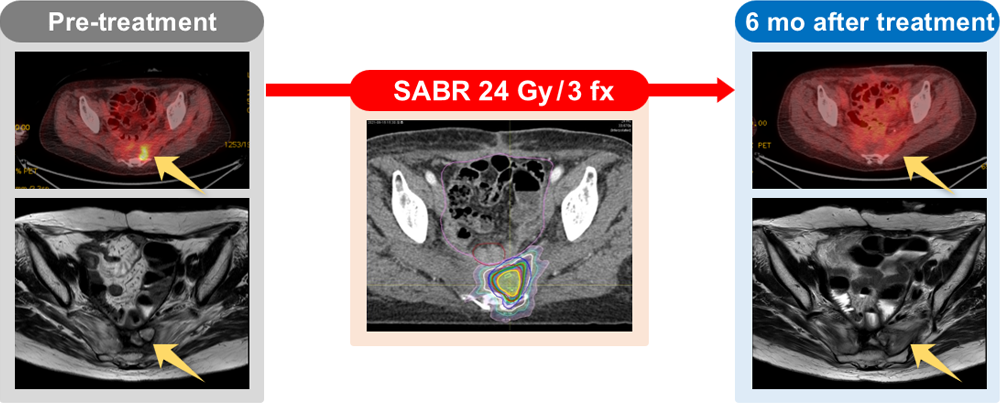
The "SABR-ROC" trial aims to evaluate the efficacy and safety of Stereotactic Ablative Radiotherapy (SABR) as a treatment modality for patients with recurrent ovarian cancer. Our objective is to explore the potential benefits of targeted radiotherapy in improving treatment outcomes and quality of life for individuals facing this challenging disease.
By participating in this trial, patients will have the opportunity to access a novel treatment approach while contributing to the advancement of medical knowledge in the field of ovarian cancer research.
If you or any of your patients are interested in participating in the "SABR-ROC" trial, I kindly request that you reach out to our research team at your earliest convenience. Our team will provide detailed information about the trial, explain the screening process, and address any questions or concerns you may have.
Your participation in this trial is crucial to its success and the potential benefits it may offer to future patients. We value your collaboration and commitment to advancing ovarian cancer treatment options.
Thank you for your attention to this invitation. We look forward to the opportunity to work together in furthering our understanding of recurrent ovarian cancer and exploring innovative treatment approaches.

Yong Bae Kim M.D. Principal Investigator, "SABR-ROC" Clinical Trial
Yonsei Cancer Center, Seoul, South Korea 
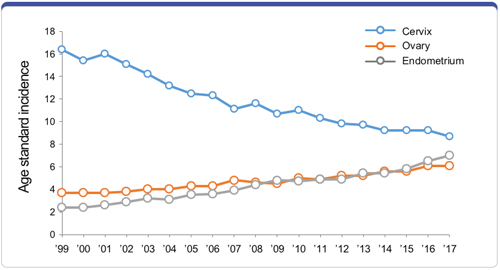
Obstet Gynecol Sci 2021
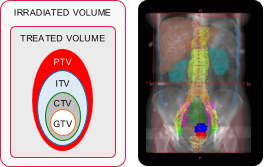
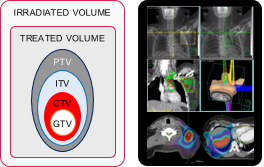
| Radiotherapy | Whole abdomen irradiation WAI | Involved field radiotherapy IFRT | Stereotactic body radiotherapy SABR |
|---|---|---|---|
| Previous studies | ㆍ1980~90s ㆍNo benefit in survival over chemoTx ㆍSerious effects |
ㆍSingle center retrospective studies ㆍProspective phase 2 ; KROG 14-05 ㆍGood efficacy and safety |
ㆍSABR-COMET; ph2, solid tumors ㆍ3 retrospective studies reported ㆍGood efficacy and safety |
| Usage status | Rarely used | Currently used often | Rapidly increasing use |
| Dose-fractions | 24 Gy / 16 fx / Additional treatment after 3 wks |
50 Gy / 25 fx / 5 wks | 24 Gy / 3 fx / 1 wk |
| Irradiated & treated volumes |
 |
 |
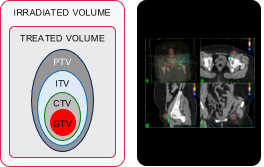 |